Background and Significance:
There is an unmet therapeutic need for patients with relapsed and/or refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL) and R/R B-cell acute lymphoblastic leukemia (B-ALL) who have failed multiple lines of therapy. PIT565 is an investigational, first-in-class, immunoglobulin G-like, trispecific antibody that targets CD19 on malignant B cells while engaging CD3 and CD2 (a costimulatory receptor) on T cells, leading to redirected T-cell cytotoxicity toward CD19-positive malignant B cells. CD2 signaling prevents transcriptional changes characteristic of exhaustion during persistent T-cell receptor stimulation of CD8 T cells. Furthermore, the loss of CD2 ligand, CD58, on tumor cells has been shown to mediate resistance to the antitumor activity by T cell-redirecting therapies, confirming the importance of CD2 activation in T cells. Findings from preclinical studies suggest that PIT565 mediates more potent and sustained antitumor T-cell responses compared with CD3 bispecifics (Lu H, ASH 2022).
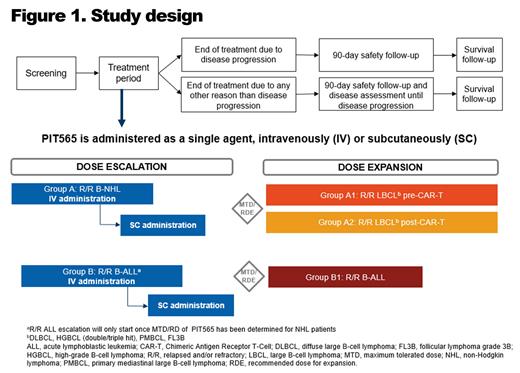
Study Design and Methods:
This is an open-label, phase 1, multicenter study of PIT565 in patients with R/R B-NHL and R/R CD19-positive B-ALL who have relapsed/failed to respond to ≥2 lines of prior therapy (including an anti-CD20 monoclonal antibody-containing chemotherapy combination regimen for patients with R/R B-NHL) (NCT05397496). The study is ongoing with plans to enroll approximately 140 patients in Belgium, France, Israel, Italy, Japan, Singapore, Spain, and the USA.
Eligible patients are ≥18 years with an Eastern Cooperative Oncology Group performance status ≤2 and have at least one bi-dimensionally measurable nodal lesion or one bi-dimensionally measurable extranodal lesion as measured via positron emission tomography-computed tomography for R/R B-NHL, and/ or morphologic disease in the bone marrow (³5% blasts) for R/R B-ALL. Patients with contraindication to tocilizumab; history of other malignant disease; active central nervous system involvement; autoimmune disease (other than patients with vitiligo, hypothyroidism only requiring hormone replacement, or psoriasis not requiring systemic treatment or conditions not expected to recur); or receiving systemic treatment with any immunosuppressive medication are not eligible.
During the dose escalation part of the study, PIT565 is administered to patients with R/R B-NHL (Group A) and patients with R/R B-ALL (Group B). During the dose-expansion part, PIT565 is administered to patients with R/R large B-cell lymphoma (LBCL) who have received CD-19-directed chimeric antigen receptor (CAR)-T therapy (Group A1); patients with R/R LBCL who have not received CAR-T therapy (Group A2); and patients with R/R B-ALL (Group B1).
PIT565 is administered either once weekly (Q1W) or once every 2 weeks (Q2W) with and without a priming dosing. Routes of administration (intravenous [IV] or subcutaneous [SC]) are explored in the dose-escalation phase. Patients initially receive PIT565 IV Q1W over a 28-day cycle. Q2W treatment in a 28-day cycle is explored if supported by preliminary pharmacokinetics (PK), pharmacodynamics, efficacy, and safety findings.
The primary objective is to assess safety (including dose-limiting toxicities) and tolerability, and to identify the maximum tolerated dose (MTD) and/or recommended dose (RD), schedule, and route of administration for each indication. Secondary objectives include evaluation of preliminary antitumor activity (overall response rate, complete response rate, best overall response, duration of response, overall survival, and progression-free survival for B-NHL or event-free survival for B-ALL), assessment of PK and immunogenicity of PIT565 in R/R B-NHL and R/R B-ALL. The dose escalation phase is guided by an adaptive Bayesian logistic regression model following the escalation with overdose control principle. The MTD and/or RD are further explored during the dose-expansion phase.
Disclosures
Avivi Mazza:AbbVie: Honoraria. Barba:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pierre-Fabre: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nektar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Palomba:Cellectar: Honoraria; Thymofox: Honoraria; Synthekine: Honoraria; BMS: Honoraria; Rheos: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Smart Immune: Honoraria; Pluto Immunotherapeutics: Honoraria; Novartis: Honoraria; GarudaTherapeutics: Honoraria; MustangBio: Honoraria; Kite: Honoraria; Ceramedix: Honoraria; Juno: Honoraria, Patents & Royalties. Alderuccio:Genentech: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Genmab: Research Funding; Abbvie: Consultancy. De Vriendt:Gilead: Consultancy, Speakers Bureau; Janssen: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Corradini:Nerviano Medical Science: Other: Honoraria (Consulting, advisory role, or lecturer); Kyowa Kirin: Other: Honoraria (Consulting, advisory role, or lecturer); Incyte: Other: Honoraria (Consulting, advisory role, or lecturer); Gilead/Kite: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Daiichi Sankyo: Other: Honoraria (Consulting, advisory role, or lecturer); Celgene: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Janssen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Novartis: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Pfizer: Other: Honoraria (Consulting, advisory role, or lecturer); Amgen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; ADC Theraputics (DSMB): Other: Honoraria (Consulting, advisory role, or lecturer); Roche: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; AbbVie: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Sanofi: Other: Honoraria (Consulting, advisory role, or lecturer); SOBI: Other: Honoraria (Consulting, advisory role, or lecturer); Takeda: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; GlaxoSmithKline: Other: Honoraria (Consulting, advisory role, or lecturer); BeiGene: Honoraria; Bristol Myers Squibb: Other: Travel and accomodations. Zinzani:JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Jain:Pfizer: Research Funding; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; CareDX: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Fate Therapeutics: Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Ipsen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; MEI Pharma: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Kite/Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Servier: Research Funding; ADC Therapeutics: Research Funding; Medisix: Research Funding; TransThera Sciences: Research Funding; Newave: Research Funding; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Takeda: Research Funding; Loxo Oncology: Research Funding; Mingsight: Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Genentech: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Novalgen: Research Funding; Dialectic Therapeutics: Research Funding; Cellectis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Aprea Therapeutics: Research Funding; Incyte: Research Funding. Leonard:Pfizer: Consultancy; Kite/Gilead: Consultancy; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Takeda: Consultancy. Coulson:Novartis: Current Employment, Current equity holder in publicly-traded company. Hassounah:Novartis: Current Employment. Yang:Novartis: Current Employment. Lu:Novartis: Current Employment. Lewandowski:Novartis: Current Employment. Polli:Novartis Institutes of Biomedical Research: Current Employment. Klopfenstein:Novartis: Current Employment. Pastore:Novartis: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Brisou:Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal